
Atom
The smallest particle of an element, which may or may not have an independent existence but always takes place in a chemical reaction is called an atom. An atom is defined as the smallest unit that retains the properties of an element. An atom is composed of sub-atomic particles and these cannot be made or destroyed. All atoms of the same element are identical and different elements have different types of atoms. Chemical reactions occur when atoms are rearranged. Atoms consist of three fundamental types of particles, protons, electrons and neutrons. Neutrons and protons have approximately the same mass and in contrast to this the mass of an electron is negligible. A proton carries a positive charge, a neutron has no charge and an electron is negatively charged. An atom contains equal numbers of protons and electrons and therefore overall an atom has no charge. The nucleus of an atom contains protons and neutrons only, and therefore is positively charged. The electrons occupy the region of space around the nucleus. Therefore, most of the mass is concentrated within the nucleus.
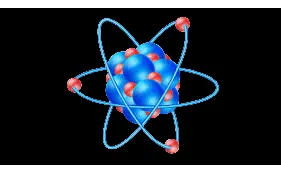
Atomic Structure
To begin with, Atomic structure of an element specify to the constitution of its nucleus and the arrangement of the electrons around it, also the atomic structure of matter is made of such as protons electrons and neutrons. atomic structure refers to the structure of an atom that containing a nucleus (centre) in which the protons (positively charged) and neutrons (neutral) are present, atomic structure and quantum mechanics dates back to the times of Democritus, He is the first person who proposed that the matter is composed of atoms. The structure of an atom gives a great insight into the entire class of chemical reactions, it bonds and also their physical properties. John Dalton proposed the first scientific theory of atomic structure in 1800s that he stated that all the atoms of an element were exactly alike, but the atoms of different elements differ in size and mass. Accordingly Dalton’s atomic theory, it involve a rearrangement of atoms to form a products. According to the postulates proposed by Dalton, the atomic structure comprises atoms, the smallest particle responsible for the chemical reaction to occur.
PARTS OF AN ATOM
PROTON
Proton is a component of an atomic nucleus and has a mass defined as one and it has a charge of +1. It is also indicated by the symbol p or p+. The number of the atomic number is equivalent to the number of protons involved in that element. Let's have a quick recap on how the proton became a proton. The word "proton" is Greek for "first". A physicist named Ernest Rutherford first used the term in 1920 to describe the nucleus of hydrogen. William Prout theorized the existence of the proton in 1815. To give you some information proton has properties. First, opposite charges attract each other which is why protons and electrons are attracted. Secondly, protons have stable particles that do not decay into other particles. Third, you can find free protons in plasma, 90% of cosmic rays consist of protons. Lastly, the radioactive decay of free neutrons which is unstable, can produce protons, electrons, and antineutrinos. For us to fully understand protons we have to prepare some examples. The nucleus in a Hydrogen atom or the H+ is an example of a proton. Each helium contains 2 protons; each lithium contains 3 protons and so on.

ELECTRON
An electron is a negatively charged subatomic particle that can be either bound to an atom or free (not bound). An electron that is bound to an atom is one of the three primary types of particles within the atom -- the other two are protons and neutrons.If you have heard of electrons you know that they have something to do with electricity and atoms. If so you are mostly right in describing what are electrons. Electrons are the subatomic particles that orbit the nucleus of an atom. They are generally negative in charge and are much smaller than the nucleus of the atom. If you wanted a proper size comparison the size of the earth in comparison to the sun would be a pretty close visualization. In the early days of atomic study, scientists believed that an atom's electrons circled the nucleus in spherical orbits at specific distances, much like planets circle a sun. In this model -- referred to as the Bohr model -- the orbits furthest from the nucleus contain the greatest amount of energy. When an electron jumps from a higher energy orbit to a lower energy orbit, the atom releases electromagnetic radiation.

NEUTRON
Two subatomic particles known as neutrons and protons are found in the nucleus of every atom. Nuetrons is one of the basic particles of the building blocks of all of us, matter and in chemistry, which is called atoms. Neutrons are somewhat heavier than positively charged protons and have an electric charge that is neither positive nor negative. Free neutrons are those that have broken away from a nucleus. These free neutrons are produced by both nuclear fusion and fission processes. The main purpose of neutrons is to act as the nuclear glue that will hold the nucleus together, this is called energy binding Neutrons are an essential tool for research in the fields of medicine, materials, and other areas. Scientists produce neutrons in research reactors and particle accelerators. Researchers project these neutrons onto samples of material. In a game of pool, some of the neutrons that collide with cue balls come into direct touch with some of the atoms.
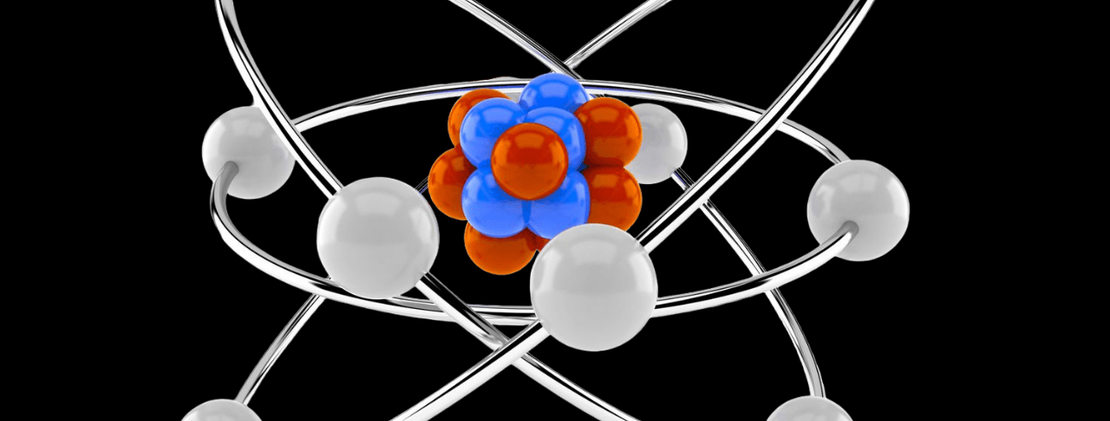
NUCLEUS
nucleus, in biology, a specialized structure occurring in most cells (except bacteria and blue-green algae) and separated from the rest of the cell by a double layer, the nuclear membrane. This membrane seems to be continuous with the endoplasmic reticulum (a membranous network) of the cell and has pores, which probably permit the entrance of large molecules. The nucleus controls and regulates the activities of the cell (e.g., growth and metabolism) and carries the genes, structures that contain the hereditary information. Nucleoli are small bodies often seen within the nucleus. The gel-like matrix in which the nuclear components are suspended is the nucleoplasm. Because the nucleus houses an organism’s genetic code, which determines the amino acid sequence of proteins critical for day-to-day function, it primarily serves as the information centre of the cell. Information in DNA is transcribed, or copied, into a range of messenger ribonucleic acid (mRNA) molecules, each of which encodes the information for one protein (in some instances more than one protein, such as in bacteria).
ELECTRON ORBIT
Electron orbitals actually are waves. On atoms, they are localized because of the electrical attraction to the nucleus, but conduction electrons are in orbitals that extend over the entire crystal. In 1957, Landauer showed that, if the length of a narrow wire connecting two bulk conductors is smaller than the size of the orbitals that carry electric current across the wire, then the electrical conductance is the same thing as wave transmission. An atom is composed of a nucleus containing neutrons and protons with electrons dispersed throughout the remaining space. Electrons, however, are not simply floating within the atom; instead, they are fixed within electronic orbitals. Electronic orbitals are regions within the atom in which electrons have the highest probability of being found. Quantum Numbers describing Electronic Orbitals There are multiple orbitals within an atom. Each has its own specific energy level and properties. Because each orbital is different, they are assigned specific quantum numbers: 1s, 2s, 2p 3s, 3p,4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p. The numbers, (n=1,2,3, etc.) are called principal quantum numbers and can only be positive numbers.
